12+ Potassium Hydroxide And Iron Ii Nitrate Precipitate
The more CrO4 2- there is the greater the quantity of precipitate that will be formed. A common example is table salt with positively charged sodium ions and negatively charged chloride ions.

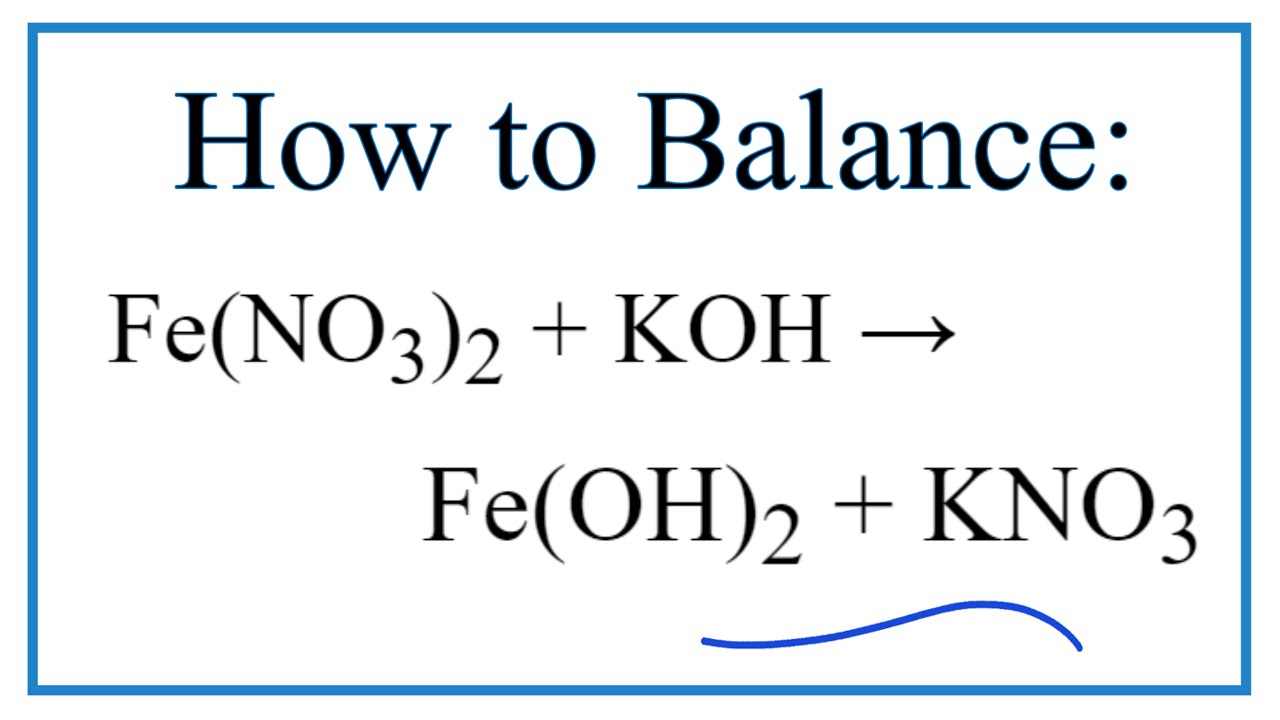
Fe No3 2 Koh Fe Oh 2 Kno3 Chemical Equation Balancer
The solid formed is called the precipitate.

. 12 13 Despite the photosensitivity of many silver compounds silver oxide is not photosensitive 14 although it readily decomposes at temperatures above 280 C. Terbium is never found in nature as a free element but it is contained in many. Reacting with potassium hexacyanoferrateII K 4 FeCN 6 solution a Procedure.
Web It will also react with solutions of alkali chlorides to precipitate silver chloride leaving a solution of the corresponding alkali hydroxide. Web IronIII oxide or ferric oxide is the inorganic compound with the formula Fe 2 O 3It is one of the three main oxides of iron the other two being ironII oxide FeO which is rare. Illustrating its hydrophilic character as much as 121 kg of KOH can dissolve in a single liter of water.
It is a rare transition metal belonging to the platinum group of the periodic tableLike the other metals of the platinum group ruthenium is inert to most other chemicals. Web Plot 2-12 By-pass Link Bweyogerere Industrial and Business Park PO Box 6329 Kampala. The primary role of the Ba2 ions in this reaction is to show the amount of CrO4 2- present.
Web Insoluble metal oxides and metal hydroxides bases will not dissolve to form an alkaline solution. A It is a displacement reaction. The clear liquid remaining.
When two solutions of ionic compounds are mixed a solid may form. Web In an aqueous solution precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. Anhydrous KOH is rarely encountered.
Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for. Has a value close to 10 14 at 25 C so the concentration of hydroxide ions in pure water is close to. K w H OH.
A Solid potassium chlorate KClO 3 decomposes to form solid potassium chloride and diatomic oxygen gas. Web In chemistry a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions which results in a compound with no net electric charge. Fe 2 aq FeCN 6 3-aq dark blue precipitate.
Bayerite doyleite and nordstranditeAluminium hydroxide is amphoteric ie it has both basic and acidic properties. But they will dissolve in acids to give the same reaction as with alkalis. Web A precipitate will form with any cation that forms an insoluble sulfate refer to the solubility rules.
In case of an inorganic chemical reaction leading to precipitation the chemical reagent causing the solid to form is called the precipitant. Web A solution of lead II nitrate is dropped into a solution of potassium iodide forming a brilliant yellow lead II iodide precipitate. C When solid sodium chloride is added to aqueous sulfuric acid hydrogen chloride gas.
Is a solution of calcium hydroxide. Web Ruthenium is a chemical element with the symbol Ru and atomic number 44. Lead Pb 2 i Add potassium iodide KI to the original solution ii Add potassium chromate K 2 CrO 4 to the original solution.
Web b i When potassium iodide solution is added to lead nitrate solution then a yellow precipitate of lead iodide is produced along with potassium nitrate solution. This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules. It is also a precipitation reaction as AgCl is a white precipitate.
The solubility of BaCr2O7 is much higher and it does not form a precipitate. Web However the Ba2 can react with the CrO4 2- to form a solid precipitate of BaCrO4. If carbon dioxide is bubbled through.
B Refer to answer 15. The component ions in a salt compound can be either. List Of Certified Products.
Web Potassium permanganate is an inorganic compound with the chemical formula KMnO 4It is a purplish-black crystalline salt that dissolves in water as K and MnO 4 an intensely pink to purple solution. Web The ammonium cation is a positively-charged polyatomic ion with the chemical formula NH 4 or NH 4 It is formed by the protonation of ammonia NH 3Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by. Web Write a balanced equation describing each of the following chemical reactions.
Ii This is a double displacement reaction. 2 g of ferrous sulphate crystals are heated in a dry boiling tube. Web Carbon dioxide reacts with calcium hydroxide solution to produce a white precipitate of calcium carbonate.
Web Honors Chemistry Name_____ Period_____ Net Ionic Equation Worksheet READ THIS. A What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride. It is a double displacement reaction.
Web 2A solution of potassium chloride when mixed with silver nitrate solution an insoluble white substance is formed. IronIII ion Fe 3 Method I. Web Aluminium hydroxide AlOH 3 is found in nature as the mineral gibbsite also known as hydrargillite and its three much rarer polymorphs.
Write the chemical reaction involved and also mention the type of the chemical reaction. Web Terbium is a chemical element with the symbol Tb and atomic number 65. I About 2 cm 3 of ironIII chloride solution is poured into a test tube.
Web Potassium hydroxide is a strong base. Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State. Web a Zinc reacts with silver nitrate to produce zinc nitrate and silver.
It is a silvery-white rare earth metal that is malleable and ductileThe ninth member of the lanthanide series terbium is a fairly electropositive metal that reacts with water evolving hydrogen gas. H 3 O OH 2H 2 O. B Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
Web It gives a yellow precipitate of compound B on treatment with iodine and sodium hydroxide solution. B Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6. Formation of a yellow precipitate for both the tests Copper Cu 2.
The hydroxide ion is a natural part of water because of the self-ionization reaction in which its complement hydronium is passed hydrogen. Ba 2 SO 4 2- BaSO 4 s Reaction with silver nitrate. On severe oxidation with potassium permanganate forms a carboxylic acid C Molecular formula C 7 H 6 0 2 which is also formed along with the yellow compound in the above.
Web Add sodium hydroxide to the original solution and treat it with Nesslers reagent K 2 HgI 4 Formation of a yellow or brown precipitate. And ironIIIII oxide Fe 3 O 4 which also occurs naturally as the mineral magnetiteAs the mineral known as hematite Fe 2 O 3 is the main source of iron for the steel industry. Add HNO 3 dropwise until solution is acidic unless of course it was dissolved in nitric acid then add a few drops of AgNO 3 and observe any reaction.
Closely related are aluminium oxide hydroxide AlOOH and aluminium. The equilibrium constant for this reaction defined as. Web The ironII ion combines with a complex ion in the reagent to produce a dark blue precipitate.
KOH reacts readily with carbon dioxide CO 2 to produce potassium carbonate K 2 CO 3 and in principle could be used to remove traces of the gas from air. Compound A does not give Tollens or Fehlings test.

Unit 4 Learning Guide Pdf Wcln Chemistry 12 Aira Flores Unit 4 Learning Guide Name Instructions Using A Pencil Complete The Following Notes As Course Hero

Maharashtra Board Class 9 Science Solutions Chapter 4 Measurement Of Matter Maharashtra Board Solutions

Self Assembled Heterometallic Complexes By Incorporation Of Calcium Or Strontium Ion Into A Manganese Ii 12 Metallacrown 3 Framework Supported By A Tripodal Ligand With Pyridine Carboxylate Motifs Stability In Their Manganese Iii Oxidized Form
How To Balance Fe No3 2 Koh Fe Oh 2 Kno3 Youtube
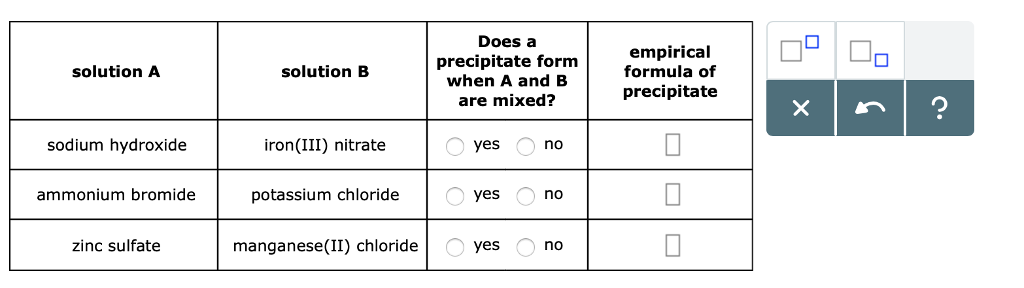
Solved Does A Precipitate Formempirical Solution A Formula Chegg Com
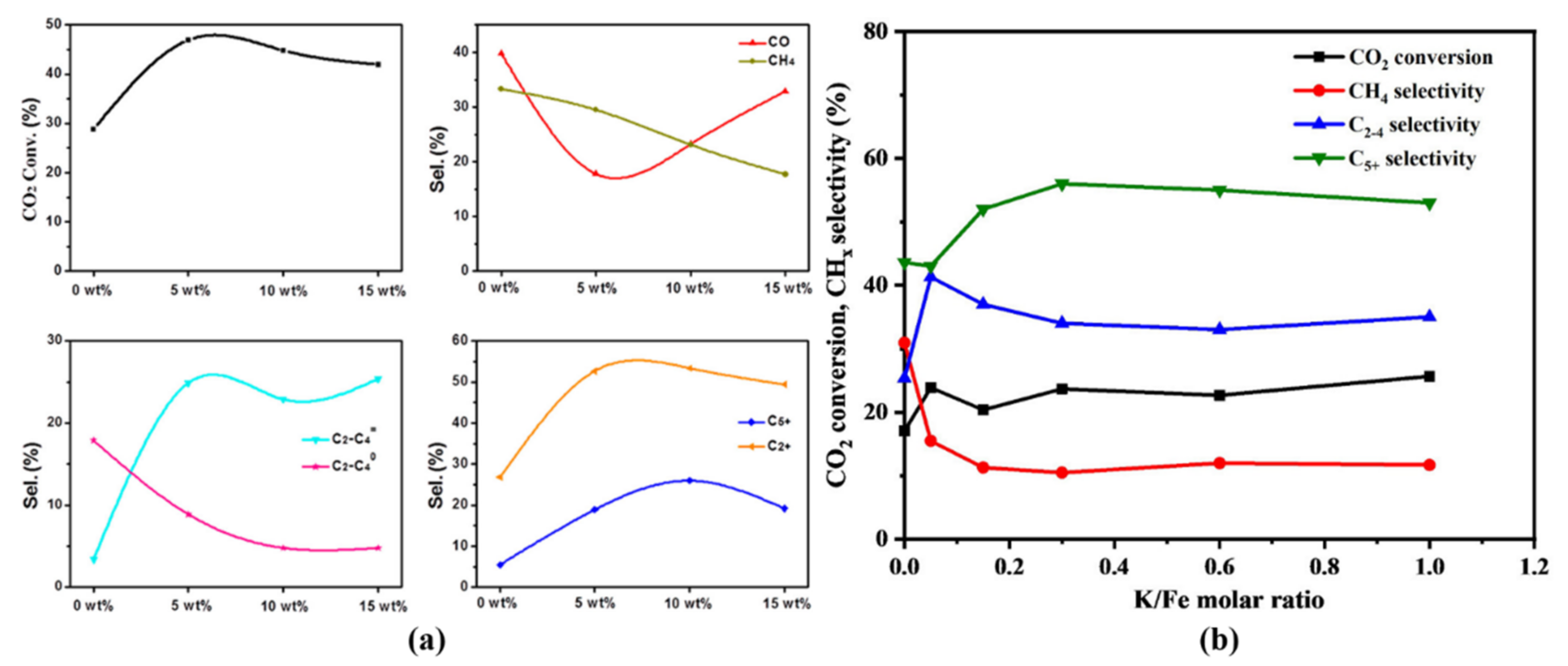
Atmosphere Free Full Text Hydrogenation Of Carbon Dioxide To Value Added Liquid Fuels And Aromatics Over Fe Based Catalysts Based On The Fischer Ndash Tropsch Synthesis Route

Cyclohexanone By Asch Issuu
Answer Page Chem Simple Science

Chemical Reactions Reactions Can Actually Be Categorized In The Following 3 Main Groupings 1 The Precipitation 2 The Redox 3 The Acid Base Synthesis Ppt Download

Pdf Chem F3 Q Ans Full Amour Rashid Academia Edu

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations Free Pdf

Chemistry Notes Form 3 All Chapters Chemistry Form 4 Spm Thinkswap

Class 11 Chemistry Chapter 12 Ncert All Solutions Pdf Archives Esaral
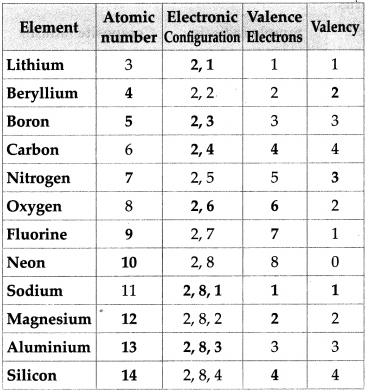
Maharashtra Board Class 9 Science Solutions Chapter 4 Measurement Of Matter Balbharati Solutions
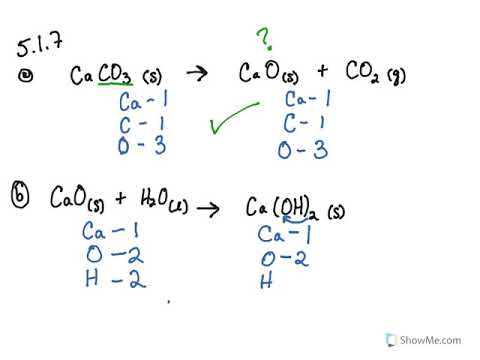
5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts
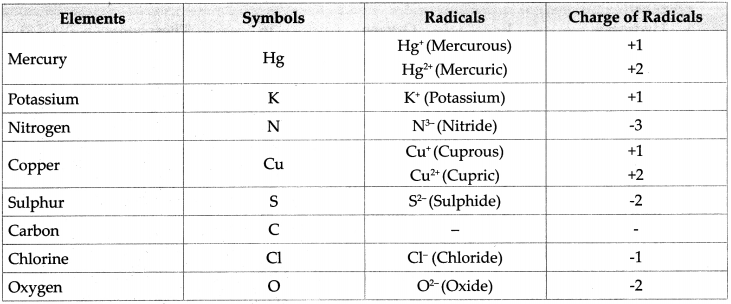
Maharashtra Board Class 9 Science Solutions Chapter 4 Measurement Of Matter Sabdekho

Us4711968a Process For The Hydrofomylation Of Sulfur Containing Thermally Cracked Petroleum Residua Google Patents